Boat ownership is a rewarding experience, offering freedom on the water and countless adventures. However, maintaining a boat requires diligence, especially when it comes to protecting its underwater metal components from corrosion. One of the most critical yet often overlooked components in this regard is the anode. Sacrificial anodes, commonly referred to as “zincs,” play a pivotal role in safeguarding your boat’s vital parts, such as propellers, shafts, and rudders, from the destructive effects of galvanic and electrolytic corrosion. This comprehensive guide explores everything you need to know about boat anodes, including their function, types, installation, maintenance, and replacement, ensuring your vessel remains in pristine condition for years to come.
What Are Boat Anodes?
Boat anodes are metallic components strategically attached to a boat’s underwater metal parts to prevent corrosion. Made from highly reactive metals like zinc, aluminum, or magnesium, anodes are designed to corrode faster than the boat’s critical components, effectively “sacrificing” themselves to protect more expensive and essential parts. This process ensures that components like the propeller, shaft, rudder, and hull remain intact, extending their lifespan and reducing the need for costly repairs.
Anodes are essential because water, particularly saltwater, acts as an electrolyte that facilitates galvanic corrosion when dissimilar metals are in contact. Without anodes, the less noble metal in the circuit—such as a bronze propeller paired with a stainless steel shaft—would corrode rapidly, leading to structural damage and compromised performance.
The Science Behind Anodes
The functionality of anodes is rooted in the principles of galvanic corrosion, an electrochemical process that occurs when two dissimilar metals are immersed in an electrolyte, such as seawater. The metals form a galvanic cell, where the less noble (more reactive) metal acts as the anode and corrodes, while the more noble metal acts as the cathode and remains protected. Sacrificial anodes are made from metals that are less noble than the boat’s components, ensuring they corrode first.
Here’s a simplified breakdown of how anodes work:
- Galvanic Cell Formation: When two metals, such as a bronze propeller and a stainless steel shaft, are submerged in water, they create a galvanic cell. The water acts as the electrolyte, allowing electrons to flow between the metals.
- Electron Sacrifice: The anode, being less noble, releases electrons, corroding in the process. This protects the more noble metal, which would otherwise corrode.
- Continuous Protection: As long as the anode remains intact and properly connected, it will continue to corrode, shielding the boat’s components.
The following table illustrates the nobility of common metals used in boating, ranked from least noble (most reactive) to most noble (least reactive):
| Metal | Nobility (Reactivity) |
|---|---|
| Magnesium | Least Noble (High) |
| Zinc | Moderately Reactive |
| Aluminum | Moderately Reactive |
| Bronze | Less Reactive |
| Stainless Steel | Most Noble (Low) |
This hierarchy explains why anodes are typically made from magnesium, zinc, or aluminum, as they corrode before bronze or stainless steel components.
Why Anodes Are Essential for Boats
Anodes are a boat’s first line of defense against corrosion, which can weaken metal components, reduce performance, and lead to costly repairs. Without anodes, underwater metals are vulnerable to two primary types of corrosion:
- Galvanic Corrosion: Occurs when two dissimilar metals are in electrical contact in an electrolyte, causing the less noble metal to corrode faster. For example, a bronze propeller paired with a stainless steel shaft will corrode without an anode.
- Electrolytic Corrosion: Caused by stray electrical currents, often from faulty wiring or nearby boats, accelerating corrosion. Anodes help mitigate this by providing a path for the current to dissipate.
The consequences of neglecting anodes are severe. A corroded propeller may lose efficiency, a damaged shaft could fail, and a compromised hull might lead to leaks. Regular anode maintenance not only preserves the boat’s structural integrity but also maintains its resale value and ensures safe operation.
Types of Boat Anodes
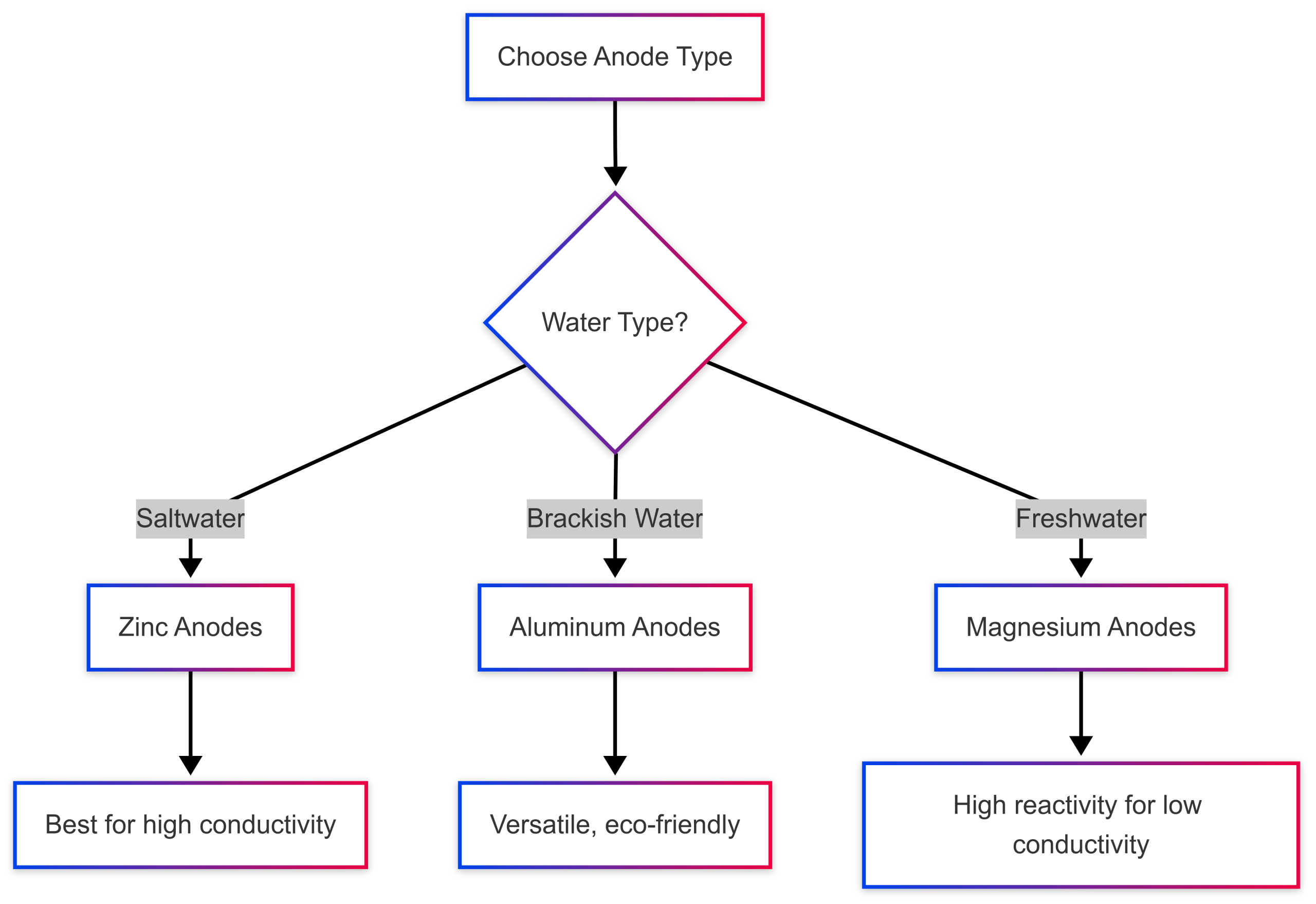
Choosing the right anode depends on the type of water your boat operates in, as different materials perform optimally in specific environments. The three primary anode materials are zinc, aluminum, and magnesium, each with distinct characteristics.
Zinc Anodes
- Best for: Saltwater environments
- Characteristics: Zinc anodes are the traditional choice for saltwater boating due to their high reactivity and effectiveness in conductive, saline water. They corrode steadily, exposing fresh layers of zinc to continue protection.
- Limitations: In freshwater or brackish water, zinc anodes can develop a passive zinc oxide film, reducing their effectiveness.
- Price Range: $10–$50 per anode, depending on size and shape.
Aluminum Anodes
- Best for: Saltwater and brackish water
- Characteristics: Aluminum anodes are lighter, longer-lasting, and more environmentally friendly than zinc. They are more active than zinc, providing protection in a wider range of water conditions, including brackish water (a mix of saltwater and freshwater).
- Limitations: Less effective in purely freshwater environments.
- Price Range: $15–$60 per anode.
Magnesium Anodes
- Best for: Freshwater environments
- Characteristics: Magnesium anodes are highly reactive, making them ideal for freshwater, where water conductivity is low. They provide robust protection against corrosion in lakes and rivers.
- Limitations: In saltwater, magnesium corrodes too rapidly, offering little protection and requiring frequent replacement.
- Price Range: $20–$70 per anode.
The following table summarizes the suitability of each anode type:
| Anode Material | Saltwater | Brackish Water | Freshwater |
|---|---|---|---|
| Zinc | ✅ | ❌ | ❌ |
| Aluminum | ✅ | ✅ | ❌ |
| Magnesium | ❌ | ❌ | ✅ |
Where Are Anodes Installed on a Boat?
Anodes are strategically placed on underwater metal components that are susceptible to corrosion. Common locations include:
- Hull: Hull plates protect the boat’s structural integrity, especially on metal boats.
- Propeller and Shaft: Zinc collars or rings are fitted to protect these critical components.
- Rudders and Trim Tabs: Disk-shaped anodes are bolted to these parts to prevent corrosion.
- Engines: Outboard and inboard engines often have anodes in cooling systems or exhaust cavities.
- Bow Thrusters: Specialized anodes protect thruster components.
- Heat Exchangers: Pencil anodes are installed in cooling systems to combat internal corrosion.
Modern boats may use a centralized active anode system, where all metal components are bonded to a single large anode, typically mounted on the transom. Proper placement ensures anodes remain submerged and in electrical contact with the protected metal.
How to Choose the Right Anode
Selecting the appropriate anode involves several considerations:
- Water Type: Match the anode material to the water environment (saltwater, brackish, or freshwater).
- Boat Size and Metal Surface Area: Larger boats or those with more underwater metal require larger or more anodes. A rule of thumb is to use an anode with a surface area of about 1% of the protected metal’s surface area.
- Component Compatibility: Ensure the anode is designed for the specific component (e.g., shaft, rudder, or hull).
- Manufacturer Recommendations: Some engines or systems require specific anode types for optimal performance.
Consulting a marine professional can help determine the correct size, shape, and material for your boat’s needs.
Installing Boat Anodes
Proper installation is critical for anodes to function effectively. Here’s a step-by-step guide:
- Locate Anodes: Identify all anode locations on your boat, including hull, propeller, shaft, rudder, and engine components.
- Remove Old Anodes: Use appropriate tools (e.g., wrench or screwdriver) to remove depleted anodes. Dispose of them responsibly, as they may contain heavy metals.
- Clean the Surface: Use a wire brush or sandpaper to ensure the mounting surface is bare and free of paint, corrosion, or debris.
- Apply Anti-Seize Compound: Coat bolts or threads with anti-seize to prevent corrosion and ease future removal.
- Secure New Anodes: Bolt or clamp the anode tightly, ensuring good electrical contact. Avoid over-tightening, which can damage components.
- Test Continuity: Use a multimeter to confirm a low-resistance connection between the anode and the protected metal.
Common Installation Mistakes
- Painting Anodes: Paint prevents anodes from corroding, rendering them useless.
- Poor Contact: Loose or corroded connections reduce effectiveness.
- Incorrect Placement: Anodes must be submerged and in direct contact with the protected metal.
Maintaining and Replacing Boat Anodes
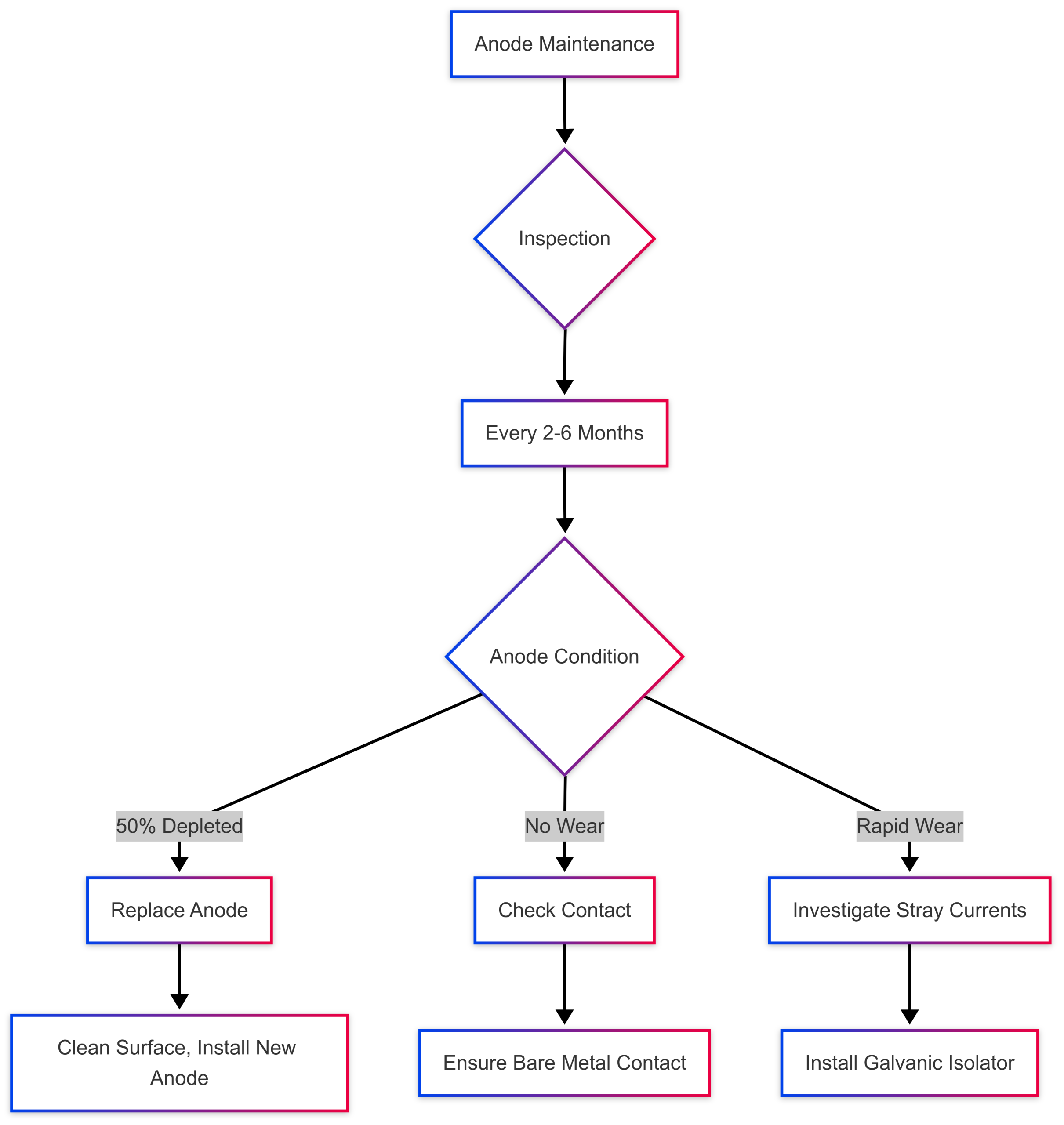
Regular maintenance ensures anodes continue to protect your boat. Follow these guidelines:
Inspection Frequency
- Saltwater: Inspect every 2–3 months due to faster corrosion rates.
- Brackish Water: Inspect every 3–4 months.
- Freshwater: Inspect every 4–6 months.
When to Replace Anodes
Replace anodes when they are approximately 50% depleted. Waiting too long leaves metal components vulnerable. Signs of replacement need include:
- Significant reduction in size or weight.
- Cracks, pitting, or excessive corrosion.
- No visible wear (indicating poor electrical contact or incorrect material).
Maintenance Tips
- Clean Connections: Ensure mounting surfaces and bolts remain free of corrosion.
- Avoid Overprotection: Too many anodes can cause overprotection, leading to excessive corrosion of other components.
- Keep a Log: Record inspection and replacement dates to track anode performance.
- Check for Hot Docks: In marinas with high electrical activity, anodes may corrode faster due to stray currents. Monitor anode wear monthly in such environments.
Replacement Costs
The cost of replacing anodes varies based on material, size, and labor (if using a professional):
- DIY Replacement: $10–$70 per anode, depending on type and size.
- Professional Service: $100–$300, including parts and labor, depending on the number of anodes and boat size.
Environmental Considerations
Anodes release metal particles into the water as they corrode, raising environmental concerns:
- Zinc vs. Aluminum: Aluminum anodes are less toxic and more environmentally friendly than zinc.
- Proper Disposal: Recycle used anodes at marinas or recycling centers to prevent heavy metal pollution.
- Regulatory Trends: Some regions are phasing out zinc anodes due to environmental concerns, favoring aluminum.
Common Anode-Related Issues and Solutions
- Anodes Not Wearing: Indicates poor electrical contact, painted anodes, or incorrect material for the water type. Check connections and replace with the appropriate anode.
- Rapid Anode Depletion: May be caused by stray currents, hot docks, or excessive electrical activity. Install a galvanic isolator to mitigate stray currents.
- Corrosion on Protected Components: Suggests depleted anodes, incorrect installation, or insufficient anode surface area. Inspect and replace anodes, and consider adding more if needed.
Advanced Anode Systems
Some modern boats use active anode systems, such as Volvo Penta’s Active Corrosion Protection System, which electronically monitors and adjusts anode performance. These systems reduce maintenance needs but require professional installation and periodic checks.
Recommended Anode Products
Here are some popular anode products and their specifications:
| Product | Material | Application | Price (USD) | Notes |
|---|---|---|---|---|
| Camp Zinc Shaft Anode | Zinc | Propeller Shaft | $15–$30 | For saltwater, various diameters |
| Martyr Aluminum Anode | Aluminum | Hull/Brackish Water | $20–$50 | Eco-friendly, long-lasting |
| Tecnoseal Magnesium | Magnesium | Freshwater Hull | $25–$60 | High reactivity for freshwater |
| Volvo Penta Bow Thruster Anode | Zinc/Aluminum | Bow Thruster | $30–$70 | Brand-specific, saltwater |
*Prices are approximate and may vary by retailer.
Conclusion
Boat anodes are indispensable for protecting your vessel from the relentless effects of galvanic and electrolytic corrosion. By understanding their role, choosing the right material for your boating environment, and maintaining them diligently, you can safeguard your boat’s underwater components, enhance its performance, and avoid costly repairs. Regular inspections, proper installation, and timely replacements are key to ensuring anodes perform effectively. Whether you’re a seasoned boater or a newcomer, prioritizing anode maintenance will keep your boat in top condition, ready for countless adventures on the water.
For professional anode replacement or to purchase high-quality anodes, contact reputable marine suppliers or service centers. Investing in anodes today is an investment in your boat’s longevity and your peace of mind.
Happy Boating!
Share ANODES: WHAT DO I NEED TO KNOW? with your friends and leave a comment below with your thoughts.
Read Severe Weather: Prepare Your Boat and Passengers until we meet in the next article.
