How Do Sacrificial Anodes Work?
Discover how sacrificial anodes protect metal structures from corrosion. Learn their function, types, applications, and benefits in this comprehensive guide.
Corrosion is a relentless adversary to metal structures exposed to harsh environments like seawater, soil, or industrial settings. From ship hulls to pipelines, corrosion can weaken critical infrastructure, leading to costly repairs or catastrophic failures. Sacrificial anodes, a cornerstone of cathodic protection, offer a robust solution to this problem. This article delves into the science, materials, applications, and practical considerations of sacrificial anodes, providing a thorough understanding of how they safeguard metal structures.
Understanding Sacrificial Anodes
What Are Sacrificial Anodes?
Sacrificial anodes, also known as galvanic anodes, are highly reactive metal components designed to corrode preferentially, protecting a less reactive metal structure from corrosion. These anodes are made from alloys with a more negative electrochemical potential than the metal they shield, such as steel or copper. By corroding in place of the protected metal, they “sacrifice” themselves, hence the name.
The primary function of a sacrificial anode is to prevent or significantly slow down the oxidation process that leads to corrosion. This is particularly critical in environments where metals are exposed to electrolytes, such as seawater or moist soil, which facilitate electrochemical reactions that degrade metal surfaces.
The Importance of Cathodic Protection
Corrosion occurs when a metal reacts with its environment, typically through an electrochemical process involving an anode (where oxidation occurs), a cathode (where reduction occurs), and an electrolyte (a conductive medium like water or soil). This reaction causes the metal to revert to its natural ore state, leading to structural weakening and eventual failure.
Cathodic protection is a technique used to control corrosion by making the protected metal structure the cathode in an electrochemical cell, thereby preventing it from oxidizing. Sacrificial anodes are one of the primary methods of cathodic protection, alongside impressed current systems and hybrid systems. Other corrosion prevention methods, such as plating, galvanization, and alloy formation, complement but do not replace the role of sacrificial anodes in many applications.
The Science Behind Sacrificial Anodes
Electrochemical Principles
Sacrificial anodes operate on the principle of galvanic corrosion, where two dissimilar metals in electrical contact and immersed in an electrolyte form a galvanic cell. The metal with the more negative electrochemical potential (the anode) corrodes preferentially, releasing electrons that protect the less reactive metal (the cathode).
For instance, consider a steel ship hull in seawater. Seawater acts as an electrolyte, and without protection, the steel would corrode as it loses electrons to the environment. By attaching a sacrificial anode, such as zinc, which has a standard reduction potential of -0.76 volts compared to steel’s -0.44 volts, the zinc corrodes instead, supplying electrons to the steel and preventing its oxidation.
This process can be visualized through a simple galvanic cell:
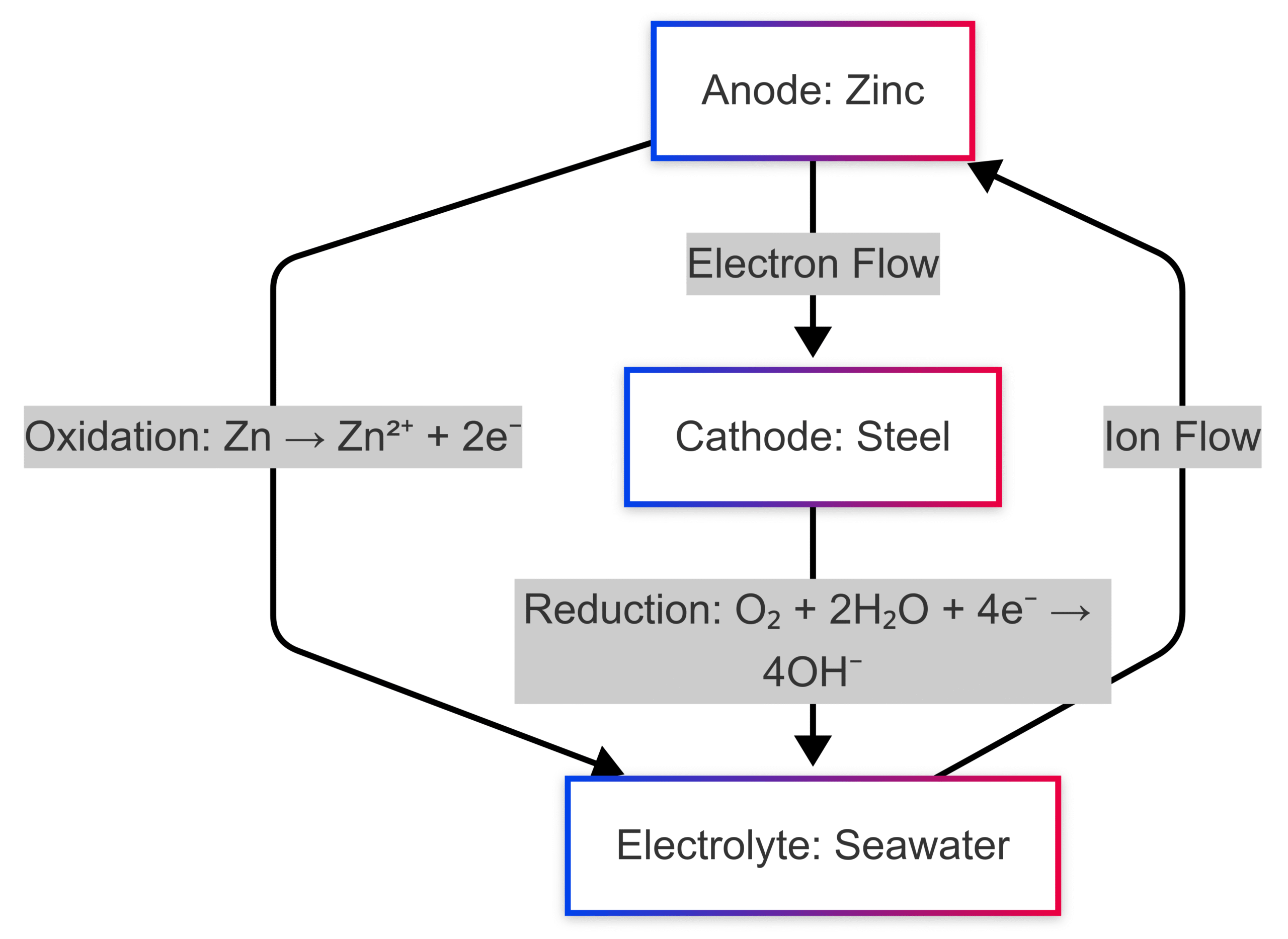
This diagram illustrates the flow of electrons from the zinc anode to the steel cathode, with oxidation occurring at the anode and reduction at the cathode, facilitated by the electrolyte.
Galvanic Corrosion Dynamics
Galvanic corrosion occurs when two metals with different electrochemical potentials are in electrical contact in the presence of an electrolyte. The more reactive metal (anode) oxidizes, releasing metal ions into the electrolyte, while the less reactive metal (cathode) remains protected. The difference in potential drives the electron flow, creating an electric current through the external circuit connecting the metals.
For example, in a marine environment, a bronze propeller on a stainless steel shaft is prone to galvanic corrosion. Without protection, the bronze would corrode rapidly. Attaching a zinc anode ensures that it corrodes instead, preserving the propeller and shaft.
Anode and Cathode Interactions
The sacrificial anode forms an electrochemical circuit with the protected metal. As the anode corrodes, it releases electrons that flow to the cathode, reducing the likelihood of oxidation on the protected metal. The rate of anode consumption depends on factors such as:
- Material Type: More reactive metals like magnesium corrode faster than zinc or aluminum.
- Surface Area: Larger anodes provide more protection but may deplete faster.
- Environmental Conditions: Higher salinity or temperature increases corrosion rates.
- Current Demand: The amount of current required to protect the cathode influences anode lifespan.
Types of Sacrificial Anodes
Sacrificial anodes are typically made from zinc, aluminum, or magnesium, each suited to specific environments and applications due to their electrochemical properties.
Zinc Anodes
Zinc anodes are the most widely used due to their high reactivity and effectiveness in marine environments. With a standard reduction potential of -0.76 volts, zinc is ideal for protecting steel structures in seawater, such as ship hulls, propellers, and rudders. Zinc anodes are available in various forms, including:
- Pencil Anodes: Cylindrical, used in pipelines and heat exchangers.
- Hull Anodes: Flat and wide, bolted to ship hulls.
- Ribbon Anodes: Flexible strips for underground applications.
Specifications:
- Potential: -0.76 V
- Current Capacity: ~780 Ah/kg
- Weight per Ampere-Year: ~10.7 kg
- Applications: Marine vessels, pipelines, underground tanks.
- Price: ~$1-$5 per kg (varies by supplier and size).
Aluminum Anodes
Aluminum anodes are preferred in both seawater and freshwater environments due to their lighter weight, longer lifespan, and lower environmental impact compared to zinc. They have a slightly less negative potential (-1.05 V) but offer higher current capacity.
Specifications:
- Potential: -1.05 V
- Current Capacity: ~2,700 Ah/kg
- Weight per Ampere-Year: ~2.9 kg
- Applications: Offshore platforms, seawater pipelines, harbor facilities.
- Price: ~$2-$6 per kg.
Magnesium Anodes
Magnesium anodes, with a potential of -1.55 V, are the most reactive and are primarily used in freshwater or low-resistivity environments like soil. Their high reactivity makes them unsuitable for prolonged use in seawater, where they corrode too quickly.
Specifications:
- Potential: -1.55 V
- Current Capacity: ~1,100 Ah/kg
- Weight per Ampere-Year: ~8.2 kg
- Applications: Water heaters, underground pipelines, storage tanks.
- Price: ~$3-$8 per kg.
Comparison Table
| Anode Type | Potential (V) | Current Capacity (Ah/kg) | Weight per Ampere-Year (kg) | Primary Applications | Price Range ($/kg) |
|---|---|---|---|---|---|
| Zinc | -0.76 | 780 | 10.7 | Marine, pipelines | 1-5 |
| Aluminum | -1.05 | 2,700 | 2.9 | Marine, freshwater | 2-6 |
| Magnesium | -1.55 | 1,100 | 8.2 | Freshwater, soil | 3-8 |
Applications of Sacrificial Anodes
Sacrificial anodes are employed across various industries to protect critical infrastructure from corrosion. Their versatility makes them indispensable in environments where metals are exposed to corrosive electrolytes.
Marine Industry
In the marine sector, sacrificial anodes are critical for protecting ship hulls, propellers, rudders, and other underwater components from galvanic corrosion in seawater. Zinc anodes are commonly used due to their effectiveness in saltwater, while aluminum anodes are gaining popularity for their longer lifespan and environmental benefits.
Example: A zinc hull anode bolted to a steel ship hull corrodes preferentially, protecting the hull from rusting. Regular inspection ensures anodes are replaced when 50% depleted, typically annually.
Oil and Gas Industry
Pipelines, offshore platforms, and storage tanks in the oil and gas industry face severe corrosion risks from both seawater and soil. Aluminum anodes are often used for offshore applications, while magnesium anodes protect buried pipelines. These anodes prevent leaks and structural failures, enhancing safety and longevity.
Example: Offshore drilling platforms use aluminum anode sleds to protect structural steel, with anodes strategically placed to ensure uniform protection.
Water Treatment Systems
In water treatment facilities, sacrificial anodes protect pipes, tanks, and heat exchangers from corrosion in freshwater environments. Magnesium anodes are preferred due to their high reactivity, while aluminum is used in systems requiring longer-lasting protection.
Example: Magnesium pencil anodes in water heaters prevent internal corrosion, extending the tank’s lifespan.
Infrastructure and Construction
Bridges, buildings, and other infrastructure exposed to moisture or soil benefit from sacrificial anodes. Zinc ribbon anodes are often used in underground applications, such as protecting steel pilings or pipelines, due to their flexibility and ease of installation.
Example: Zinc anodes buried alongside a steel pipeline prevent corrosion, with backfill materials ensuring consistent current output.
Installation and Maintenance
Installation Methods
Sacrificial anodes are attached to structures using lead wires, cast-in straps, or direct bolting. Proper electrical contact is critical to ensure effective protection:
- Lead Wires: Welded or mechanically connected to the structure, insulated to prevent resistance loss.
- Cast-in Straps: Welded or bolted, ensuring low-resistance contact.
- Direct Bolting: Common for hull plates, requiring clean, bright metal surfaces for optimal conductivity.
For buried anodes, a conductive backfill (e.g., gypsum or bentonite) enhances current distribution and anode performance.
Maintenance Requirements
Sacrificial anodes require regular inspection and replacement to maintain protection. Key considerations include:
- Inspection Frequency: Typically annual, or more frequent in harsh environments.
- Replacement Threshold: Anodes should be replaced when 50% depleted to ensure continuous protection.
- Environmental Factors: High temperatures or salinity accelerate anode consumption, necessitating more frequent checks.
Maintenance Workflow:
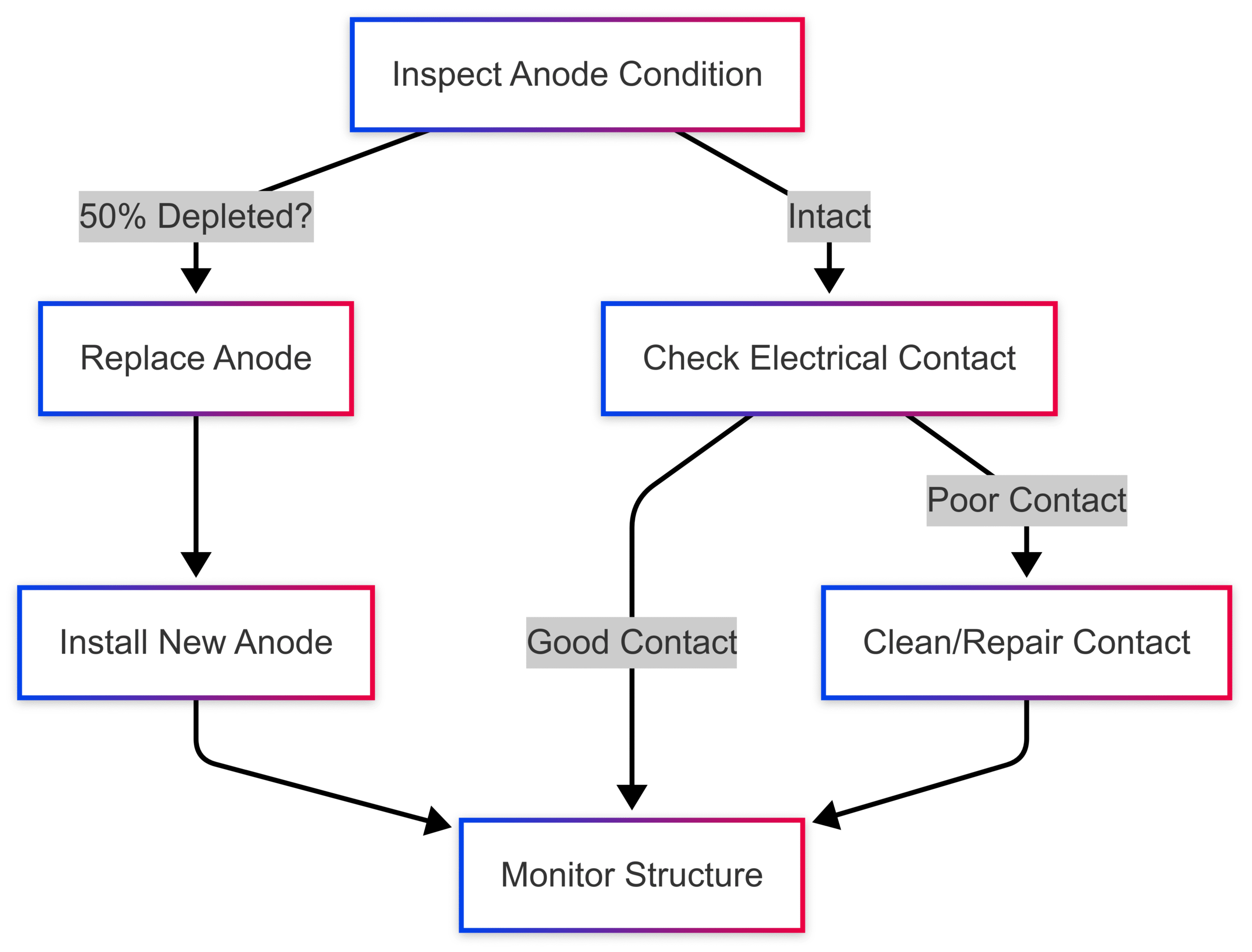
Challenges and Considerations
- Biofilm Formation: In marine environments, biofilms can alter anode performance, potentially reversing protection (e.g., copper alloys protected by aluminum anodes may corrode if biofilms form unevenly).
- Material Selection: Choosing the wrong anode material (e.g., magnesium in seawater) can lead to rapid depletion or insufficient protection.
- Spark Hazards: Magnesium anodes in hydrocarbon-containing environments (e.g., oil tanker ballast tanks) pose explosion risks if detached.
Advantages and Disadvantages
Advantages
- Simplicity: No external power source required, making installation and operation straightforward.
- Reliability: Self-regulating current output reduces maintenance needs.
- Robustness: Anodes are durable and resistant to mechanical damage in most applications.
Disadvantages
- Weight: Large systems require significant anode mass, increasing structural load.
- Limited Lifespan: Anodes must be replaced periodically, adding to maintenance costs.
- Environmental Restrictions: Magnesium anodes are unsuitable for certain applications due to safety concerns.
Comparison with Impressed Current Systems
Sacrificial anodes are often compared to impressed current cathodic protection (ICCP) systems, which use inert anodes and an external power source. The following table highlights key differences:
| Feature | Sacrificial Anode System | Impressed Current System |
|---|---|---|
| Power Source | None | External DC power |
| Complexity | Simple | Complex |
| Weight | High for large systems | Lower |
| Maintenance | Periodic replacement | Regular monitoring |
| Applications | Marine, pipelines | Large offshore structures |
| Cost | Lower initial, higher maintenance | Higher initial, lower maintenance |
Hybrid systems, combining sacrificial and impressed current anodes, are used in complex structures like the Murchison and Hutton platforms to balance weight and protection needs.
Practical Considerations for Boat Owners
For boat owners, sacrificial anodes are critical for protecting underwater components like propellers, rudders, and hulls. Key tips include:
- Sizing: Use anodes with sufficient surface area (e.g., 1% of the protected metal’s surface area) to ensure adequate protection.
- Material Choice: Use zinc or aluminum in saltwater, magnesium in freshwater.
- Installation: Ensure metal-to-metal contact and avoid painting anodes, as this prevents electron flow.
- Inspection: Check anodes annually and replace when 50% depleted.
Example Calculation:
To protect a steel hull with a surface area of 100 m² in seawater:
- Current Demand: ~110 mA/m² (bare steel) = 11 A total.
- Zinc Anode Weight: 11 A × 10.7 kg/A-year ≈ 118 kg for one year of protection.
- Replacement Schedule: Replace when 50% consumed, typically every 6-12 months.
Environmental and Economic Considerations
Aluminum anodes are increasingly preferred due to their lower environmental impact compared to zinc, which contains trace amounts of cadmium. Aluminum is less toxic and has a higher current capacity, reducing replacement frequency. However, zinc remains cost-competitive in many applications due to its lower initial cost.
Cost Breakdown:
- Zinc Anodes: $100-$500 for a typical boat, depending on size and number of anodes.
- Aluminum Anodes: $150-$700, with longer lifespan offsetting higher initial costs.
- Magnesium Anodes: $200-$800, primarily for freshwater or buried applications.
Conclusion
Sacrificial anodes are a vital tool in the fight against corrosion, protecting critical infrastructure across marine, oil and gas, water treatment, and construction industries. By leveraging the electrochemical properties of reactive metals like zinc, aluminum, and magnesium, these anodes sacrifice themselves to preserve the integrity of larger metal structures. Understanding their function, proper installation, and maintenance ensures long-term protection and cost savings. Whether you’re a boat owner or an engineer managing offshore platforms, sacrificial anodes are an indispensable ally in extending the lifespan of metal assets.
Happy Boating!
Share How Do Sacrificial Anodes Work? with your friends and leave a comment below with your thoughts.
Read GT52 vs GT54 – Unleashing the Ultimate Sonar Revo until we meet in the next article.






